Refractory Gout Management
- English
- Español
Pathogenesis
Refractory gout is considered an uncommon problem, but it remains a persistent challenge, and patients with this condition can have functional impairment and sharply reduced quality of life.1
Gout may result from chronic hyperuricemia, defined as a serum urate level >7.0 mg/dL in men and >6.0 mg/dL in women. Hyperuricemia is generally due to overproduction or renal under-excretion of uric acid, and ultimately leads to urate crystal deposition. Hyperuricemia is associated with metabolic syndrome, diabetes, and hypertension, all of which increase in prevalence with age.2, 3, 4 Certain medications can increase uric acid levels.5 Hyperuricemia is also strongly associated with chronic kidney disease (CKD).6,7
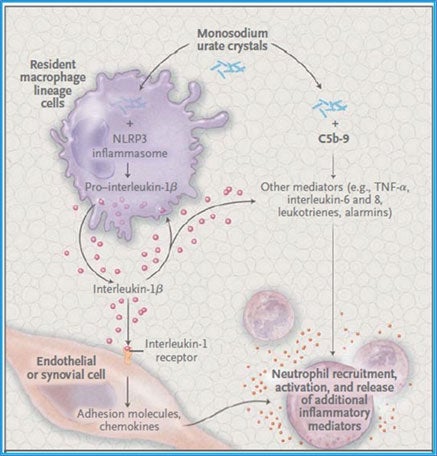
The initial phase of gout is characterized by intermittent acute inflammatory arthritic attacks that can resolve over a period of 7 to 10 days. The mechanisms of inflammation in gout are summarized in Figure 1. Various peripheral joints can be affected, but the first metatarsophalangeal (MTP) joint is the most commonly impacted. If inadequately treated, transition to the second phase can occur, manifested as chronic gout, characterized by tophaceous deposits of urate crystals (tophi) in soft tissues of joints, and ultimately chronic joint destruction, leading to increasing pain and risk of disability. Many individuals (40% - 60%) experiencing a first gouty attack experience a second gout attack within one year.8
Refractory gout (also known as treatment failure gout) is a clinical condition that is characterized by serum uric acid levels that do not fall below 6 mg/dL, and ongoing symptoms of recurrent flares, chronic inflammatory arthritis, and increased tophi.1
Despite available therapies, the inability to manage the disease and maintain serum uric acid levels <6 mg/dL remains a significant challenge.10 An estimated 3% - 10% of patients with gout in the United States are not adequately managed.10, 11 Many of these issues are directly or indirectly related to the use of urate-lowering medications. Factors can include delayed prescribing, inadequate dosage, nonadherence, intolerance, or inadequate response despite maximum doses that are otherwise tolerated. Despite the availability of therapies, there remains a subset of patients with gout who, despite aggressive therapy, have intractable disease (i.e., patients with refractory gout).1,10,11
Diagnosis
It is important that patients with gout be accurately diagnosed because the disease may be associated with significant morbidity and many patients may require lifetime therapy. Diagnosis involves the measurement of serum uric acid levels, and examination of the signs and symptoms associated with gout. A more definitive diagnosis is based on the presence of monosodium urate crystals in joint aspiration fluid.12, 13
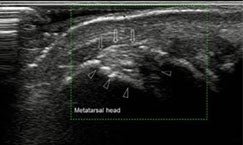
When gout cannot be established through histological crystal diagnosis, then imaging tools can help establish or confirm a diagnosis. Imaging can also be used to assess the severity and burden of the inflammatory and structural impact of gout.14 Imaging tools and techniques utilized in gout can include conventional radiography, ultrasound, computed tomography, dual energy computed tomography, magnetic resonance imaging, and nuclear medicine.14 Ultrasound-detected erosions of gout in the first MTP joint are shown in Figure 2. In addition to diagnosis, imaging can be used to monitor response to treatment. If serum urate levels do not fall below 6 mg/dL and symptoms persist, despite treatment, then refractory gout should be considered.
Treatment
Acute Gout
The main therapies for acute gout are aimed at timely relief of pain and disability caused by intense inflammation. Options for managing acute attacks include the use of nonsteroidal anti-inflammatory drugs (NSAIDs), colchicine, glucocorticoids, and possibly corticotropin. NSAIDs and colchicine are considered the first-line agents for acute attacks, but if poorly tolerated or contraindicated, glucocorticoids or corticotropin may be employed.15, 16
However, treatment selection and dosing should be individualized and based on the patient's clinical circumstances and the presence of comorbid conditions, such as CKD. For example, NSAIDs can negatively impact kidney function. Prolonged use of NSAIDs is not recommended in patients with glomerular filtration rate (GFR) <60 mL/min/1.73m2. NSAIDs should be avoided in patients with GFR <30 mL/min/1.73m2.17,18 Many patients with CKD also have diabetes, so their blood sugar levels can be negatively impacted with the use of glucocorticoids.
Chronic and Refractory Gout
Therapies aimed at prevention of future attacks and management of chronic gout include reducing risk factors, dietary and lifestyle interventions, and urate-lowering therapy.
Patient education regarding diet, lifestyle, treatment objectives, and management of comorbidities is a core therapeutic measure in gout.19,20,21 Dietary recommendations to manage chronic gout can include limiting alcohol (particularly beer), meat and seafood, and foods/beverages high in fructose. Adequate water intake and prevention of dehydration can also be helpful in preventing gout attacks, although fluid restrictions for certain patients (e.g., dialysis, heart failure) need to be considered.
Long-term prophylaxis with urate lowering therapy is used to maintain serum urate levels below 6 mg/dL, while considering the individual needs of the patient. Xanthine oxidase inhibitors (XOIs), uricosuric, and uricase agents are three classes of drugs approved for lowering urate levels to help prevent acute flares and development of tophi in patients with gout.
Allopurinol and febuxostat are XOIs that inhibit urate synthesis and are considered first-line agents for chronic gout.15,16,22 Allopurinol is generally used at a dose of 300 mg/day (maximum dose 800 mg/day) with adjustments required in patients with kidney disease. The CONFIRMS trial was a Phase III study that found that febuxostat 80 mg was more efficacious than febuxostat 40 mg and allopurinol 300 mg/200 mg in achieving a serum urate level <6.0 mg/dL at the final visit and was safe in patients with mild-to-moderate CKD.23
If the disease is refractory to monotherapy with either of XOI option, then a uricosuric agent can be added to an XOI as a second-line approach.15 Uricosuric drugs (including probenecid and lesinurad) block renal tubular urate reabsorption. These drugs can be used in patients with under excretion of urate, but are generally not recommended in patients with advanced kidney disease.
Pegloticase, a modified recombinant uricase, is available for chronic gout that is refractory to conventional treatment.24 It is administered intravenously every 2 weeks. Studies have shown its efficacy in lowering urate levels and reducing tophi in this patient subpopulation.22,24 A study by Edwards et al analyzed data from two randomized clinical trials.25 The study included refractory gout patients with or without clinically apparent tophi, and showed a significant clinical benefit (uric acid lowering and/or improvement of symptoms) with over 6 months of treatment with pegloticase. A post hoc analysis by Yood et al showed that the use of pegloticase did not adversely affect eGFR, regardless of CKD stage.26
The optimal management of refractory gout has been an ongoing challenge despite the availability of multiple therapeutic options.27,28 This healthcare challenge is being further complicated by the association of hyperuricemia with other chronic conditions including CKD, diabetes and hypertension. Adequate diagnosis, timely treatment of acute attacks, optimal management of chronic gout, and appropriate adjustment of treatment regimens for cases of refractory gout can offer the best opportunities to improve patient outcomes.
References
- Fels E, Sundy J. Refractory gout: what is it and what to do about it? Curr Opin Rheumatol. 2008;20:198-202.
- Doherty M. New insights into the epidemiology of gout. Rheumatology (Oxford). 2009;48(suppl 2):ii2-ii8.
- Terkeltaub R. Update on gout: new therapeutic strategies and options. Nat Rev Rheumatol. 2010;6:30-38.
- Wertheimer A, Morlock R, Beck MA. A revised estimate of the burden of illness of gout. Curr Thera Res. 2013;75:1-4.
- Moriwaki Y. Effects on uric acid metabolism of the drugs except the antihyperuricemics. J Bioequiv Availab. 2014;6:10-17.
- Johnson R, Nakagawa T, Jalal D, Sánchez-Lozada L, Kang D, Ritz E. Uric acid and chronic kidney disease: which is chasing which? Nephrol Dial Transplant. 2013;28: 2221–2228.
- Iseki K, Ikemiya Y, Inoue T, et al. Significance of hyperuricemia as a risk factor for developing ESRD in a screened cohort. Am J Kidney Dis. 2004;44:642-650.
- Halpern R, Fuldeore M, Mody R, et al. The effect of serum urate on gout flares and their associated costs. J Clin Rheumatol. 2009;15:3-7.
- Neogi T. Clinical practice. Gout. N Engl J Med. 2011;364:443-452.
- Ali S, Lally E. Treatment Failure Gout. Med Health RI. 2009;92:369-371.
- Edwards N. Treatment-failure gout: a moving target. Arthritis Rheum. 2008;58:2587-2590.
- Zhang W, Doherty M, Pascual E, et al. EULAR evidence based recommendations for gout. Part I: Diagnosis. Report of a task force of the Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis. 2006; 65:1301-1311.
- Zhang W, Doherty M, Bardin T, et al. EULAR evidence based recommendations for gout. Part II: Management. Report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis. 2006;65:1312-1324.
- Chowalloor P, Siew T, Keen H. Imaging in gout: A review of the recent developments. Ther Adv Musculoskelet Dis. 2014;6:131-143.
- Khanna D, Fitzgerald J, Khanna P, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res. 2012;64:1431-1446.
- Khanna D, Khanna P, Fitzgerald J, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 2: therapy and antiinflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res. 2012;64:1447-1461.
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl. 2013;3:1-150
- Perazella M, Shirali A. Kidney disease caused by therapeutic agents. In: Gilbert S, Weiner D, Gipson D, Perazella M, Tonelli M., eds. National Kidney Foundation. Primer on kidney diseases. 6th ed. Philadelphia, PA: Saunders Elsevier; 2014.
- Choi H. A prescription for lifestyle change in patients with hyperuricemia and gout. Curr Opin Rheumatol. 2010;22:165-172.
- Choi HK, Atkinson K, Karlson EW, et al. Alcohol intake and risk of incident gout in men: a prospective study. Lancet. 2004;363:1277-1281.
- Choi HK, Atkinson K, Karlson EW, et al. Purine-rich foods, dairy and protein intake, and the risk of gout in men. N Engl J Med. 2004;350:1093-1103.
- Sriranganathan M, Vinik O, Falzon L, et al. Interventions for tophi in gout: a Cochrane systemic literature review. J Rheumatol Suppl. 2014;92:63-69.
- Becker M, Schumacher H, Espinoza L, et al. The urate-lowering efficacy and safety of febuxostat in the treatment of the hyperuricemia of gout: the CONFIRMS trial. Arthritis Res Ther. 2010;12:R63.
- Shannon J, Cole S. Pegloticase: a novel agent for treatment-refractory gout. Ann Pharmacother. 2012;46:368-376.
- Edwards N, Singh J, Troum O, et al. THU0448. Characterization of patients with chronic refractory gout who do and do not have clinically apparent tophi: response to pegloticase. Ann Rheum Dis. 2017;76:377.
- Yood R, Ottery F, Irish W, et al. Effect of pegloticase on renal function in patients with chronic kidney disease: a post hoc subgroup analysis of 2 randomized, placebo-controlled, phase 3 clinical trials. BMC Research Notes. 2014;7:54-60.
- Doherty M, Jansen TL, Nuki G, et al. Gout: why is this curable disease so seldom cured? Ann Rheum Dis. 2012;71:1765-1770.
- Perez-Ruiz F, Carmona L, Yebenes MJ, et al. An audit of the variability of diagnosis and management of gout in the rheumatology setting: the gout evaluation and management study. J Clin Rheumatol. 2011;17:349-355.
