Vaccines are one of the most effective tools to protect your health and prevent disease. Vaccines work with your body’s natural defenses so your body will be ready to fight the virus if you are exposed (also called immunity).
While the effectiveness rates of COVID-19 vaccines are very good, we now know that people who are on immunosuppression medications for the treatment of advanced kidney disease and kidney transplant recipients, may not receive the same level of protection, also known as antibody immunity, from the COVID-19 vaccine as people who are not on immunosuppressive medication.
While more research is needed to learn more about the effectiveness in people with advanced CKD, those on dialysis, and transplant recipients -- these vaccines have been demonstrated to be safe in this population.
The National Kidney Foundation urges patients with advanced kidney disease, including transplant and dialysis patients and patients requiring immunosuppression for treatment of advanced kidney disease, to continue masking and practicing social distancing, after being fully vaccinated.
These vaccines cannot give you COVID-19.
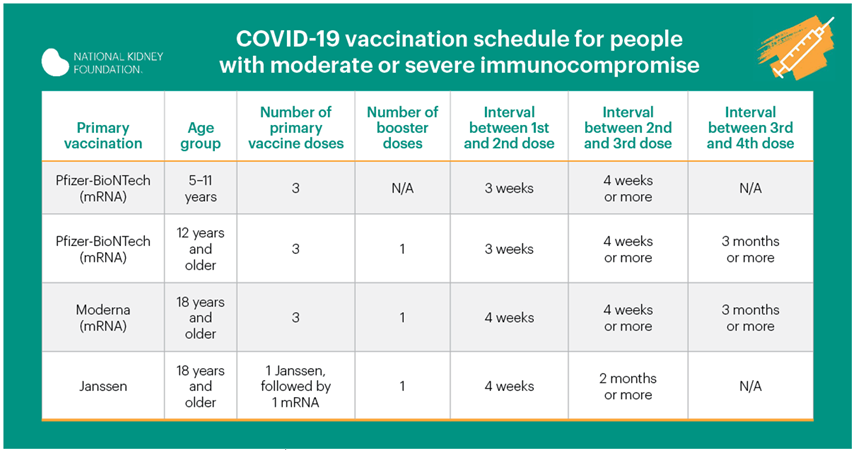
Vaccine second booster for immune suppressed
The U.S. Food and Drug Administration (FDA) has authorized the use of a second booster of COVID-19 mRNA vaccine from either Pfizer-BioNTech or Moderna in patients who are immunocompromised (have a weak immune system). Patients with organ transplants, and others who are immunocompromised, should get an dose mRNA booster of COVID-19 vaccine after having received three doses and first booster. Using a different brand of mRNA vaccine is allowed for the booster if the brand previously received is not available.
Patients do not need a prescription or approval from a health care provider to prove they are immunocompromised to receive the second booster. For now, the FDA has determined that others who are fully vaccinated do not need a second booster.
Find a COVID-19 vaccine or booster:
- Search vaccines.gov
- Text your ZIP code to 438829
- Call 1-800-232-0233 to find locations near you.
COVID-19 testing
Vaccine types, explained
Viral vector vaccines
The COVID-19 vaccine from Johnson & Johnson is a viral vector vaccine, which uses a harmless version of a different virus, called a “vector,” to deliver information to your body's immune systems to help protect you from COVID-19.
How do viral vector vaccines work?
The vaccine teaches your body how to make copies of COVID-19 spike proteins. If you are exposed to the real virus later, your body will recognize it and know how to fight it off. The vaccine DOES NOT contain the virus that causes COVID-19 and cannot give you COVID-19. It also cannot make you sick from the virus that is used as the vector. It cannot change your DNA in any way.
mRNA vaccines
Both the Pfizer/BioNTech and Moderna COVID-19 vaccines are messenger RNA vaccines – also called mRNA vaccines, and they are a new type of vaccine to protect against infectious diseases.
To trigger an immune response, many vaccines put a weakened or inactivated germ into our bodies. Other vaccines can use a particle or tiny part of the germ to trigger an immune response.
But – mRNA vaccines do not work this way. Instead, they teach our cells how to make a protein – or even just a piece of a protein – which triggers an immune response inside our bodies. This immune response produces antibodies – and antibodies are what protect us from getting infected if the real virus enters our bodies.
Have mRNA vaccines been studied?
At this time, the only licensed mRNA vaccines in the United States are the ones from Pfizer/BioNTech and Moderna. However, researchers have been studying and working with them for decades.
Interest has grown in these vaccines because they can be developed in a laboratory using readily available materials. This means the process can be standardized and scaled up, making vaccine development faster than traditional ways of making vaccines.
mRNA vaccines have been studied before for other viruses including the flu, Zika, rabies, and cytomegalovirus (CMV). As soon as the necessary information about the virus that causes COVID-19 was available, scientists began designing the mRNA instructions for cells to build the unique spike protein into an mRNA vaccine.
Future mRNA vaccine technology may allow for one vaccine to provide protection for multiple diseases, thus decreasing the number of shots needed for protection against common vaccine-preventable diseases. mRNA vaccines have also been studied for use in cancer.
Facts vs fiction
"I can get COVID-19 from the COVID-19 vaccine."
FALSE.
None of the COVID-19 vaccines in the United States contain the live virus that causes COVID-19. This means that a COVID-19 vaccine cannot make you sick with COVID-19.
"I’ve already had COVID-19, so I don’t need to get a vaccine."
FALSE.
Due to the severe health risks associated with COVID-19 and the fact that re-infection with COVID-19 is possible, you should get the vaccine even if you’ve already had COVID-19.
The immunity someone gains from having an infection, called natural immunity, varies from person to person. At this time, it is unknown how long immunity may last.
"A COVID-19 vaccination will help protect me from getting COVID-19."
TRUE.
COVID-19 vaccination works by teaching your immune system how to recognize and fight the virus that causes COVID-19, and this protects you from getting sick with COVID-19.
"The COVID-19 vaccine will change my DNA."
FALSE.
The two vaccines that are currently available for adults in the United States, do not change or interact with your DNA in any way. None of the ingredients in a COVID-19 vaccine enters the nucleus of the cell, which is where our DNA is kept.
This means the mRNA cannot affect or interact with our DNA in any way. Instead, COVID-19 mRNA vaccines work with the body’s natural defenses to safely develop immunity to disease.
"I shouldn’t get vaccinated because I may want children someday."
FALSE.
People who want to get pregnant in the future should get vaccinated. Based on current knowledge, experts believe that COVID-19 vaccines are unlikely to pose a risk to a person trying to become pregnant in the short or long term.
Scientists study every vaccine carefully for side effects immediately and for years afterward. The COVID-19 vaccines are being studied carefully now and will continue to be studied for many years, similar to the ways in which other vaccines that come to market.
"I should continue wearing a mask when I am vaccinated."
TRUE.
If you are immunocompromised, you should keep wearing a mask after you are vaccinated to help protect yourself and others from getting COVID-19.
Source: CDC
COVID-19 variants
Information about the characteristics of new COVID-19 variants is rapidly emerging. Scientists are working to learn more about how easily they spread, if they cause more severe illness, and if COVID-19 vaccines will protect people against them.
What we know
Viruses, including COVID-19, constantly change through mutation, such as the Delta and Omicron variants, and new COVID-19 variants are expected to continue to occur. People living with kidney disease at any stage and kidney transplant recipients may not have as much protection against COVID-19, even if they are fully vaccinated. The NKF recommends following the same CDC guidelines for people who are not fully vaccinated, including wearing a mask in public indoor spaces. Sometimes new variants emerge and disappear. Other times, new variants emerge and persist. Multiple variants of the virus that causes COVID-19 have been documented in the United States and globally during this pandemic.
The virus that causes COVID-19 is a type of coronavirus, and can be identified by crown-like spikes found on their surfaces. Scientists monitor changes in the virus, including changes to the spikes on the surface of the virus. These studies, including genetic analyses of the virus, are helping scientists understand how changes to the virus might affect how it spreads and what happens to people who are infected with it.
Side effects
After receiving a COVID-19 vaccination, you may have some side effects. This is a normal sign that your body is building up protection from the virus.
The side effects from COVID-19 vaccination may feel like the flu and might even affect your ability to do some daily activities, but symptoms should go away in a few days. Some of the common side effects include:
- On the arm where you got the shot:
- Pain
- Swelling
- Throughout the rest of your body:
- Fever
- Chills
- Tiredness
- Headache
- To reduce pain and discomfort where you got the shot:
- Apply a clean, cool, wet washcloth over the area
- Use or exercise your arm
Contact your doctor or healthcare provider if your:
- Your symptoms worsen, such as if redness and tenderness increase at the spot on your arm where you got your shot
- Side effects are worrying you and are not going away after a few days
- While mild or moderate side effects are fairly common with the COVID-19 vaccines, do not be alarmed if you do not have any side effects at all.
According to the World Health Organization (WHO), there are many people who do not have any side effects after receiving their COVID-19 vaccine. No reaction doesn’t mean the vaccine is is not working. It just means everybody responds differently to the vaccination.
Keep in mind, both Pfizer-BioNTech and Moderna have reported that aside from minor pain at the injection site, only about half of the people in clinical trials reported any side effects after vaccination.
Allergic reactions
There have been reports that some people have experienced severe allergic reactions – also known as anaphylaxis – after getting a COVID-19 vaccine. As an example, an allergic reaction is considered severe when a person needs to be treated with epinephrine or an EpiPen© or if they must go to the hospital.
There have also been reports that some people have experienced non-severe allergic reactions within 4 hours after getting vaccinated (known as immediate allergic reactions), such as hives, swelling, and wheezing (respiratory distress).
The CDC recommends that people with a history of severe allergic reactions – not related to vaccines or injectable medications, such as foods, animals, venom, environmental, or latex allergies – should get vaccinated. People with a history of allergies to oral medications or a family history of severe allergic reactions should also get vaccinated.
Severe allergic reactions
If you have had a severe allergic reaction to any ingredient in a COVID-19 vaccine, you should not get vaccinated. If you had a severe allergic reaction after getting the first dose of either the Pfizer-BioNTech or Moderna COVID-19 vaccine, CDC recommends that you should not get the second dose.
Non-severe allergic reactions
If you have had an immediate allergic reaction—even if it was not severe—to any ingredient in an mRNA COVID-19 vaccine, CDC recommends that you should not get the Pfizer-BioNTech or Moderna COVID-19 vaccines. If you had an immediate allergic reaction after getting the first dose of an mRNA COVID-19 vaccine, you should not get the second dose. Your doctor may refer you to a specialist in allergies and immunology to provide more care or advice.
While severe allergic reactions to the Johnson & Johnson vaccine are very rare, if you have had a severe allergic reaction to any of the ingredients in the vaccine, you should not get the shot.
Allergy safety recommendations
The CDC has recommendations for COVID-19 vaccination providers about how to prepare for the possibility of a vaccine recipients having a severe allergic reaction:
- All people who get a COVID-19 vaccine should be monitored on site
- People who have had severe allergic reactions or who have had any type of immediate allergic reaction to a vaccine or injectable therapy should be monitored for at least 30 minutes after getting the vaccine
- All other people should be monitored for at least 15 minutes after getting the vaccine
- Vaccination providers should have appropriate medications and equipment – such as epinephrine (EpiPen©), antihistamines, stethoscopes, blood pressure cuffs, and timing devices to check your pulse – at all COVID-19 vaccination sites
If you experience a severe allergic reaction after getting a COVID-19 vaccine, vaccination providers should provide rapid care and call for emergency medical services. You should continue to be monitored in a medical facility for at least several hours.
Ingredients by provider
Pfizer-BioNTech COVID-19 vaccine ingredients:
mRNA (messenger ribonucleic acid), lipids ((4-hydroxybutyl)azanediyl)bis(hexane-6,1-diyl)bis(2-hexyldecanoate), 2 [(polyethylene glycol)-2000]-N,N-ditetradecylacetamide, 1,2-Distearoyl-sn-glycero-3-phosphocholine, and cholesterol), potassium chloride, monobasic potassium phosphate, sodium chloride, dibasic sodium phosphate dihydrate, and sucrose
Moderna COVID-19 vaccine ingredients:
mRNA (messenger ribonucleic acid), lipids (SM-102, polyethylene glycol [PEG] 2000 dimyristoyl glycerol [DMG], cholesterol, and 1,2-distearoyl-sn-glycero-3-phosphocholine [DSPC]), tromethamine, tromethamine hydrochloride, acetic acid, sodium acetate, and sucrose
Johnson & Johnson COVID-19 vaccine ingredients:
Recombinant, replication-incompetent adenovirus type 26 expressing the SARS-CoV-2 spike protein, citric acid monohydrate, trisodium citrate dihydrate, ethanol, 2-hydroxypropyl-β-cyclodextrin (HBCD), polysorbate-80, sodium chloride.
Effectiveness rates
While the effectiveness rates of COVID-19 vaccines are very good, we now know that people who are on immunosuppression medications for the treatment of advanced kidney disease and kidney transplant recipients, may not receive the same level of protection, also known as antibody immunity, from the COVID-19 vaccine as people who are not on immunosuppressive medication.
Most doctors agree that the benefits of the vaccine for people with chronic kidney disease at any stage, those on dialysis, and kidney transplant recipients are much greater than the risk of serious disease or complications from COVID-19. Talk to your doctor or other healthcare professional about getting a COVID-19 vaccine.
Safety
The United States Vaccine Safety System ensures that all vaccines are as safe as possible. Even though kidney transplant recipients were not included in early COVID-19 clinical trials, many doctors believe the COVID-19 vaccine is safe for these patients.
While more research is needed to learn more about the effectiveness in people with advanced CKD, those on dialysis, and transplant recipients -- these vaccines have been demonstrated to be safe in this population.
Most doctors agree that the benefits of the vaccine for people with chronic kidney disease at any stage, those on dialysis, and kidney transplant recipients are much greater than the risk of serious disease or complications from COVID-19. Talk to your doctor or other healthcare professional about getting a COVID-19 vaccine.
Blood clots have occurred in some people who have received the Johnson and Johnson COVID-19 vaccine. The chance of having this happening is considered very rare (1 in ~500,000). The clots involved blood vessels in the brain, abdomen, and legs along with low levels of platelets (blood cells that help your body stop bleeding).
In people who developed these blood clots and low levels of platelets, symptoms began approximately one to two weeks following vaccination. Most people who developed these blood clots and low levels of platelets were females ages 18 - 49 years.
Following these reports, the FDA and CDC underscored the confidence in the vaccine’s safety and effectiveness, following a data assessment. Available data suggested potential blood blots were rare events.
People should seek medical attention right away they have any of the following symptoms after receiving the Johnson and Johnson COVID-19 vaccine:
• Shortness of breath,
• Chest pain
• Leg swelling
• Persistent abdominal pain
• Severe or persistent headaches or blurred vision
• Easy bruising or tiny blood spots under the skin beyond the site of the injection
Source: FDA/CDC announcement (https://www.fda.gov/news-events/press-announcements/fda-and-cdc-lift-recommended-pause-johnson-johnson-janssen-covid-19-vaccine-use-following-thorough)
Join our free Q&A webinar and Facebook Live, COVID-19 Vaccine: What Kidney Patients Need to Know, on Jan 11th, 8 am PT/ 11 am ET.