Introduction
Despite significant advances in managing dialysis patients, there remains a critical need to improve care within this population due to poor outcomes and survival.
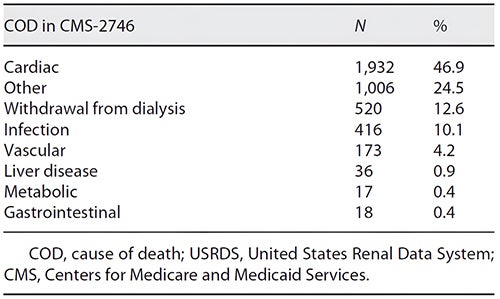
Improvements have occurred in managing co-morbidities including cardiovascular disease, anemia, bone-mineral disease, fluid overload, diabetes, and hypertension. However, as demonstrated by United States Renal Data System (USRDS) and Centers for Medicare and Medicaid Services (CMS) data, there is minimal positive impact of current dialysis modalities on clinical outcomes, with more than 50% of dialysis patients dying from cardiovascular causes and infections. (Table 1)
Table 1: Causes of death in dialysis
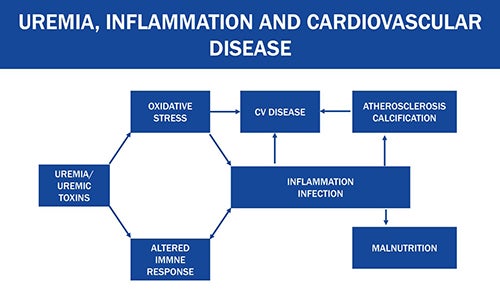
Research has established that uremic toxins negatively impact outcomes and quality of life in dialysis patients, despite current dialytic methods. The middle molecular toxins have shown associations with inflammation, cardiovascular disease, infection, altered immune response, and malnutrition.
Figure 1: Uremia, inflammation, and cardiovascular disease
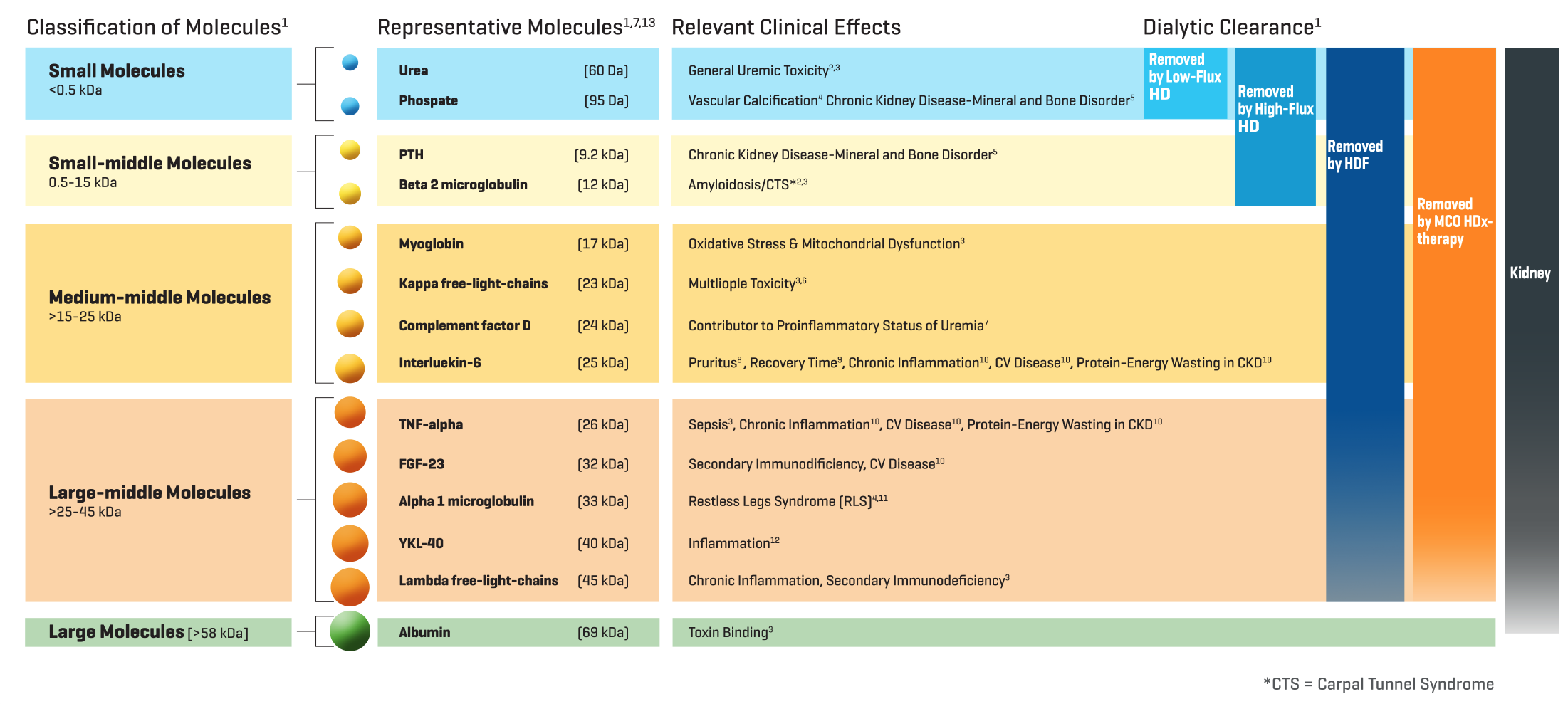
An expert consensus statement has described the associations of middle molecule uremic toxins with clinical symptoms and outcomes, including pruritus, restless legs, long recovery time from dialysis treatments, and poor quality of life. (Figure 2) Current HD modalities such as low flux and high flux have limited capacity to remove middle molecular uremic toxins. Thus, there is a strong mandate to further increase the removal of middle molecular toxins to improve the quality of life and survival in HD patients.
Since the HD dialyzer membrane acts as the artificial kidney that clears uremic toxins, it should replicate as closely as possible the toxin clearing capacity of the native kidney. Based upon current evidence, medium cut- off (MCO) dialyzer membranes closely mimic the clearance profile of the native kidney. (Figure 2) This bulletin provides an overview of MCO dialyzers, their role in expanded hemodialysis (HDx), and the clinical impact of this dialytic approach.
Figure 2: Uremic toxins by class and linkage with clinical symptoms and outcomes
Overview of Medium Cut-Off Dialyzers
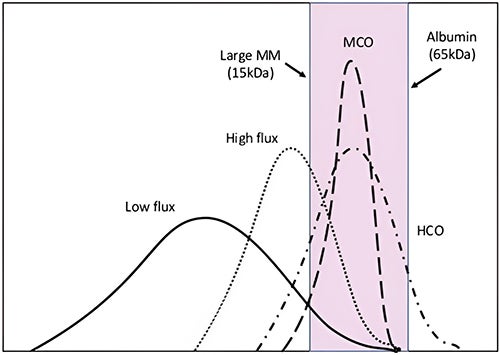
MCO dialyzers are now available for use in conventional HD settings. MCO membranes have larger pores than high flux membranes which significantly improves middle molecule clearance, along with a unique structure which retains essential proteins.1,2 MCO membranes are made of polyarylethersulfone/polyvinylpyrrolidone and have a tighter pore distribution with larger pores compared to high-flux and low-flux membranes.3 The pores have a radius between 3 and 3.5 nm after contact with blood, and a mean pore radius of 5 nm.4 (Figure 3) This porosity results in an adjustment between the molecular weight retention onset and molecular weight cut-off of the membranes, allowing greater removal of the larger middle molecular uremic toxins (25–58 kDa) with controlled albumin clearance.5 (Figure 4)
Figure 3: Main features of medium cut-off dialyzer4
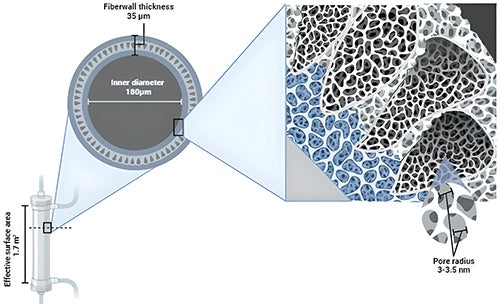
Figure 4: Pore size distribution in dialysis membranes
The primary mechanism of MCO dialyzers is diffusion, but with an increased internal filtration rate due to the reduced internal diameter of the fibers of the MCO dialyzer and the increased fiber length that enhances the convective volume inside the dialyzer.5,7 The reduced thickness and inner diameter of the fibers improves membrane permeability and dialysis efficiency because there are more fibers in a more compact dialyzer, thereby increasing the wall shear rate and optimizing blood flow.3,8 MCO is the dialyzer with the smallest inner diameter in the capillary (180 nm), enabling augmented internal filtration. This principle occurs mainly in the distal part of the dialyzer, compensating for the filtration achieved in the proximal part without need for reinfusion, as in hemodiafiltration (HDF).3,9 The ultrafiltration control system of the dialysis machine regulates the process, providing the exact amount of net filtration required for the prescribed weight loss.3,10
In summary, the MCO membrane has four characteristics which distinguish it from dialyzers used in high flux HD: increased permeability due to large pore size enables improved clearance of large middle molecular toxins; asymmetric pore size distribution improves selectivity through stable separation; adsorption provides safety and effectiveness against contaminants; smaller internal diameter allows enhanced removal of large middle molecular toxins.
Because of the expanded molecular weight range of uremic toxins removed, the term “expanded HD (HDx)” refers to HD performed with MCO dialyzers. Unlike hemodiafiltration (HDF), HDx employs conventional dialysis machines without specific software or replacement fluid, while using standard parameters (blood flow ≥ 300 mL/min and a dialysate flow 500 mL/min).11,12 These features make HDx an important step toward individualized care that may improve outcomes and quality of life for more people on HD.
Concept of Expanded Hemodialysis
Conventional HD removes small molecule uremic toxins through diffusion, but it has limited ability to remove middle molecules and protein-bound uremic toxins.13 And though high-flux membranes increase middle molecule clearance, they also have increased permeability due to larger pore size and an increased ultrafiltration coefficient,3 leading to controlled and limited albumin removal. Therefore, removal of protein-bound uremic toxins may be limited,3,14 and not significantly improved in comparison to low flux membranes.15 While HDF combines diffusion and convection to enhance clearance of middle molecule uremic toxins it requires specialized dialysis machines for generation of sterile substitution fluid and staff trained to operate them, which are not available in many dialysis units.13 This also requires additional dialysate quality monitoring. Studies of both HDF and HDx have utilized treatment times of at least 4 hours,16 making time a potential implementation barrier for dialysis clinics and patients, particularly in the U.S. where 72.1% of the population had session durations less than 4.0 hours in 2023 USRDS data.
HDx achieves a high level of clearance for molecules such as β-2 microglobulin and free light chains, (molecular weight of 22.5 and 45 kDa for kappa and lambda, respectively).17 Although albumin losses are documented at 1.2 to 3.5 g per dialysis session,18 hepatic synthesis of albumin in the setting of normal liver function may be compensatory. A positive feature of albumin leakage is that it may promote removal of protein-bound uremic toxins such as indoxyl sulfate and p-cresyl sulfate that are not otherwise removed due to their binding to albumin, despite their low molecular weight (< 500 Da). Moreover, the removal of inflammatory cytokines (IL-6, TNF-α) and other toxins may be an added value of HDx.3,19,20
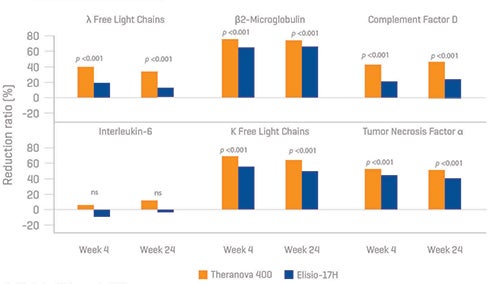
Studies have focused on the efficacy and safety of MCO dialyzers compared to conventional HD and/or HDF. In a meta-analysis of nine studies comparing MCO dialyzers with high-flux dialyzers, MCO dialyzers resulted in higher clearance of middle molecules (e.g., beta-2-microglobulin) and lower levels of tumor necrosis factor-alpha.21 A systematic meta-analysis of 18 prospective interventional studies with a total of 853 dialysis patients confirmed the safety and efficacy of MCO membranes compared to high flux-HD (increased reduction ratio of β-2microglobulin, kappa and lambda free light chains)—these effects were not greater compared to HDF, but notably, there were no significant differences in albumin loss compared to HDF.22 In a randomized controlled trial by Weiner et al. of 172 HD patients, use of an MCO dialyzer showed an increased reduction rate of both kappa and lambda free chains, complement factor D, IL 6 and tumor necrosis factor α compared to standard high flux-HD.23 (Figure 5) Similar results were also found in two other randomized controlled trials.24,25
Figure 5: Efficacy and safety of expanded hemodialysis
Clinical Impact of Expanded Hemodialysis
Uremic toxins are associated with physical symptoms, such as fatigue, itching, and restless legs syndrome, leading to a reduced quality of life, which could be improved with effective toxin removal. A prospective, multicenter observational study of 992 patients found that 3 of 5 domains from the Kidney Disease Quality of Life 36-Item Short Form Survey (KDQoL-SF36) improved after changing from high-flux HD to HDx for 12 months. The number of patients with restless legs syndrome was significantly reduced at 12 months (22% vs. 10%, p < 0.001).3,26 In a study of 49 HD patients randomized to either a MCO membrane or high-flux membrane, the MCO group reported higher scores in the physical functioning and physical role domains of KDQoL-SF36, with lower scores for morning pruritus and less scratching during sleep.3,27
Studies have also compared HDx to high-flux membranes and/or HDF in terms of effects on inflammation and oxidative stress markers associated with endothelial dysfunction, vascular calcification, increased cardiovascular risk, malnutrition and mortality.17,20,24,25 One study reported reduced expression of pro-inflammatory TNF-α and IL-6 mRNA in peripheral leukocytes in patients treated with MCO membranes compared to high-flux membranes, although cytokine levels during 12 weeks of follow-up were not significantly different.20 Kim et al. investigated the change in the large-middle molecule removal rate, which is associated with vascular calcification, when using a MCO dialyzer compared to a high-flux dialyzer. Results from the group of 20 patients showed that the reduction ratios of FGF23, OPG, and sclerostin were significantly higher when using the MCO dialyzer than the high- flux dialyzer.28 The most recent randomized controlled trial (RCT) examining the effects of HDF vs HDx on uremic toxin clearance included 40 patients and concluded that pre-dialysis toxin levels at the end of the study were similar between groups. HDF showed greater removal of uremic toxins, while HDx was comparable to HDF in maintaining pre-dialysis levels of middle molecules and inflammatory cytokines.12 Regarding cardiovascular outcomes, an RCT by Lee et al. showed there were no differences in cardiovascular parameters such as echocardiography, changes in brachial-ankle pulse wave velocity between HDx and HDF, although the coronary artery calcium score over 1 year increased in the HDx group.29 In a crossover trial of 81 patients treated in three clinics, HDx was associated with cost savings compared with high-flux HD, including fewer hospitalizations and reduced erythropoietin use. All patients were treated with high-flux HD for at least one year before switching to HDx for an additional year.30
An observational multicenter study involving 1098 dialysis patients over 2-year period showed lower all cause hospitalization incidence rate for HDx with MCO dialyzers compared to high flux hemodialysis. Non-fatal cardiovascular events were lower in patients treated by HDx with MCO dialyzers.31 Additionally, less use of erythropoietin-stimulating agents and iron supplementation has been reported with HDx, suggesting that improved removal of inflammatory mediators may improve iron metabolism and erythropoietin-stimulating agent resistance.32 Evidence supporting the promise of HDF includes a 2023 New England Journal of Medicine RCT comparing HDF to high flux HD with over 600 patients in each arm treated at 61 European clinics that showed HDF statistically significantly reduced mortality 33% (hazard ratio 0.77; 95% confidence interval 0.65-0.93) and improved quality of life parameters over a median follow up of 30 months.33
Summary
The efficacy and safety of MCO dialyzers have been confirmed in multiple studies, with MCO dialyzers demonstrating superiority to high-flux dialyzers for larger middle molecule clearance and reducing markers of inflammation and oxidative stress, while minimizing loss of albumin at a level comparable to HDF. Using MCO dialyzers to perform HDx is an innovative dialytic approach with potential broad applicability in HD units with existing infrastructure.13 HDx offers a greater range of uremic toxin clearance that can potentially improve patient quality of life and outcomes and is of particular importance where HDF is not available, when HDF is not indicated, or is too costly to implement.
References
- 1. Schmidt RJ, Flythe JE. Overview of the hemodialysis apparatus. UptoDate. Accessed November 7, 2024. Topic 1887 Version 32.0.
- 2. Cho NJ, Park S, Islam MI, et al. Long-term effect of medium cut-off dialyzer on middle uremic toxins and cell-free hemoglobin. PLoS One 2019; 14:e0220448.
- 3. Fiorentino, M., La Fergola, F. & De Rosa, S. Medium cut-off dialyzer for middle molecular uremic toxins in AKI and chronic dialysis. J Nephrol 2024;37:23–37.
- 4. Del Toro-Cisneros N, Zuñiga-González EY, Caballero-Islas AE, Geraldo-Murillo JA, Arvizu-Hernández M, Vega-Vega O. Are medium cut-off membranes the future, or the promising reality for chronic hemodialysis patients? Rev Invest Clin 2023;75(6):289-299.
- 5. García-Prieto A, de la Flor JC, Coll E, Iglesias E, Reque J, Valga F. Expanded hemodialysis: what’s up, Doc? Clin Kidney J. 2023;16:1071-1080.
- 6. Wolley M, Jardine M, Hutchison CA. Exploring the Clinical Relevance of Providing Increased Removal of Large Middle Molecules. Clinical Journal of the American Society of Nephrology. Clin J Am Soc Nephrol. 2018;13:805-814.
- 7. Kirsch AH, Lyko R, Nilsson LG et al. Performance of hemodialysis with novel medium cut-off dialyzers. Nephrol Dial Transplant 2017;32:165–72.
- 8. Jonny J, Teressa M. Expanded hemodialysis: a new concept of renal replacement therapy. J Investig Med. 2023;71:38–41.
- 9. Ronco C, Marchionna N, Brendolan A, et al. Expanded haemodialysis: from operational mechanism to clinical results. Nephrol Dial Transplant. 2018;33:iii41–iii47.
- 10. Lorenzin A, Neri M, Lupi A, et al. Quantification of internal filtration in hollow fiber hemodialyzers with medium cut-off membrane. Blood Purif. 2018;46:196–204.
- 11. Lukkanalikitkul, E., Kidkaem, H., Phonrat, M. et al. A randomized trial comparing medium cut-off membrane dialyzers with online hemodiafiltration for uremic toxins clearance in hemodialysis patients. Sci Rep 2025;15:5467.
- 12. Heguilén RM, Sciurano C, Bellusci AD, et al. The faster potassium-lowering effect of high dialysate bicarbonate concentrations in chronic haemodialysis patients. Nephrol Dial Transplant 2005; 20:591.
- 13. Lu C, Wang Y, Wang D, et al. Hypomagnesemia and Short-Term Mortality in Elderly Maintenance Hemodialysis Patients. Kidney Dis 2020; 6:109.
- 14. van Gelder MK, Middel IR, Vernooij RWM, et al. Protein-bound uremic toxins in hemodialysis patients relate to residual kidney function, are not influenced by convective transport, and do not relate to outcome. Toxins 2020;12:234.
- 15. Lesaffer G, De Smet R, Lameire N, et al. Intradialytic removal of protein-bound uraemic toxins: role of solute characteristics and of dialyser membrane. Nephrol Dial Transplant. 2000;15:50–57.
- 16. Lindgren A, Fjellstedt E, Christensson A. Comparison of Hemodialysis Using a Medium Cutoff Dialyzer versus Hemodiafiltration: A Controlled Cross-Over Study. Int J Nephrol Renovasc Dis. 2020 Oct 27;13:273-280.
- 17. Cozzolino M, Magagnoli L, Ciceri P, Conte F, Galassi A. Effects of a medium cut-off (Theranova®) dialyser on haemodialysis patients: a prospective, cross-over study. Clin Kidney J 2021;14:382–389.
- 18. Maduell F, Broseta JJ, Rodriguez-Espinosa D, et al. Comparison of four medium cut-off dialyzers. Clin Kidney J 2022;15:2292–2299.
- 19. Yeter HH, Korucu B, Derici K, et al. Effects of medium cut-off dialysis membranes on inflammation and oxidative stress in patients on maintenance hemodialysis. Int Urol Nephrol 2020;52:1779–1789.
- 20. Zickler D, Schindler R, Willy K, et al. Medium cut-off (MCO) membranes reduce inflammation in chronic dialysis patients-a randomized controlled clinical trial. PLoS ONE 2017;12:e0169024.
- 21. Yang J, Ke G, Liao Y, et al. Efficacy of medium cut-off dialyzers and comparison with high-flux dialyzers in patients on maintenance hemodialysis: A systematic review and meta-analysis. Ther Apher Dial 2022; 26:756.
- 22. Zhao Y, Gan L, Niu Q, Ni M, Zuo L. Efficacy and safety of expanded hemodialysis in hemodialysis patients: a meta-analysis and systematic review. Ren Fail 2022;44:541–550.
- 23. Weiner DE, Falzon L, Skoufos L, et al. Efficacy and safety of expanded hemodialysis with the Theranova 400 dialyzer: a randomized controlled trial. Clin J Am Soc Nephrol 2020;15:1310–1319.
- 24. Belmouaz M, Bauwens M, Hauet T, et al. Comparison of the removal of uraemic toxins with medium cut-off and high-flux dialysers: a randomized clinical trial. Nephrol Dial Transplant 2020;35:328–335.
- 25. Sevinc M, Hasbal NB, Yilmaz V, et al. Comparison of circulating levels of uremic toxins in hemodialysis patients treated with medium cut-off membranes and high-flux membranes: Theranova in sisli hamidiye etfal (THE SHE) randomized control study. Blood Purif 2020;49:733–742.
- 26. Alarcon JC, Bunch A, Ardila F, et al. Impact of medium cut-off dialyzers on patient-reported outcomes: COREXH registry. Blood Purif. 2021;50:110–118.
- 27. Lim J-H, Park Y, Choi S-Y, et al. Randomized controlled trial of medium cut-off versus high-flux dialyzers on quality of life outcomes in maintenance hemodialysis patients. Sci Rep. 2020;10:7780.
- 28. Kim HJ, Seong EY, Song SH. Medium cut-off dialyzer improves reduction ratios of large middle molecules associated with vascular calcification. Kidney Res Clin Pract. 2024;43:753-762.
- 29. Lee Y, Jang M, Jeon J, et al. Cardiovascular risk comparison between expanded hemodialysis using Theranova and online hemodiafiltration (CARTOON): a multicenter randomized controlled trial. Sci Rep. 2021;11:10807.
- 30. Sanabria RM, Hutchison CA, Vesga JI, et al. Expanded hemodialysis and its effects on hospitalizations and medication usage: a cohort study. Nephron. 2021;145:179–187.
- 31. Molano AP, Hutchison CA, Sanchez R, et al. Medium Cutoff Versus High-Flux Hemodialysis Membranes and Clinical Outcomes: A Cohort Study Using Inverse Probability Treatment Weighting. Kidney Medicine, Volume 4, Issue 4, 100431.
- 32. Lim J-H, Jeon Y, Yook JM, et al. Medium cut-off dialyzer improves erythropoiesis stimulating agent resistance in a hepcidin-independent manner in maintenance hemodialysis patients: results from a randomized controlled trial. Sci Rep 2020;10:16062.