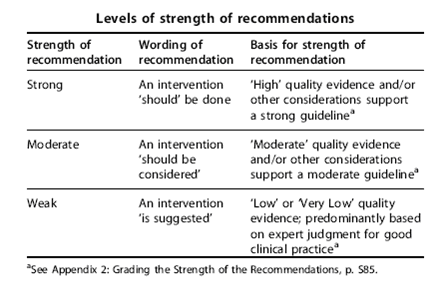
Hepatitis C Management and Hemodialysis
Hepatitis C virus (HCV) infection is associated with increased mortality among patients on hemodialysis (HD).
Prevalence of HCV infection in the HD population:
- Varies worldwide from 1% to more than 70%
- In the USA, is overall 14% and 10-fold higher than in the general population
- Is highly variable between units within the same country
Total time spent on dialysis is among the risk factors for the presence of anti-HCV antibodies and/or HCV RNA. HCV is the major etiologic agent of chronic hepatitis and possible liver cirrhosis and hepatocarcinoma.
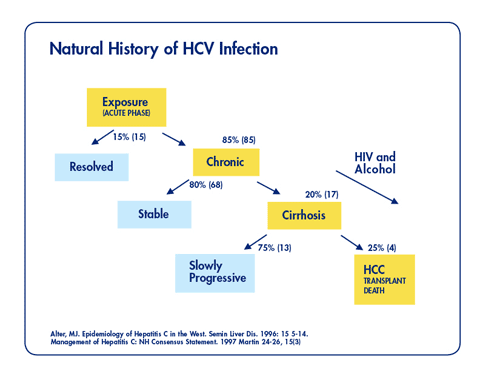
Note: Little is known about the natural history of HCV in the chronic kidney disease population and if it differs substantially from those with normal kidney function.
This reference tool highlights select guidelines from the KDIGO Clinical Practice Guidelines for the Prevention, Diagnosis, Evaluation and Treatment of Hepatitis C in Chronic Kidney Disease for implementation in the U.S. and in accordance with KDOQI U.S. Commentary on the KDIGO Clinical Practice Guideline for the Prevention, Diagnosis, Evaluation, and Treatment of Hepatitis C in CKD.
Based on KDIGO Clinical Practice Guidelines for the Prevention, Diagnosis, Evaluation, and Treatment of Hepatitis C in Chronic Kidney Disease. To view the full guideline publication, visit www.kdigo.orgDetection and Evaluation of HCV in Chronic Kidney Disease (Guideline 1.2)
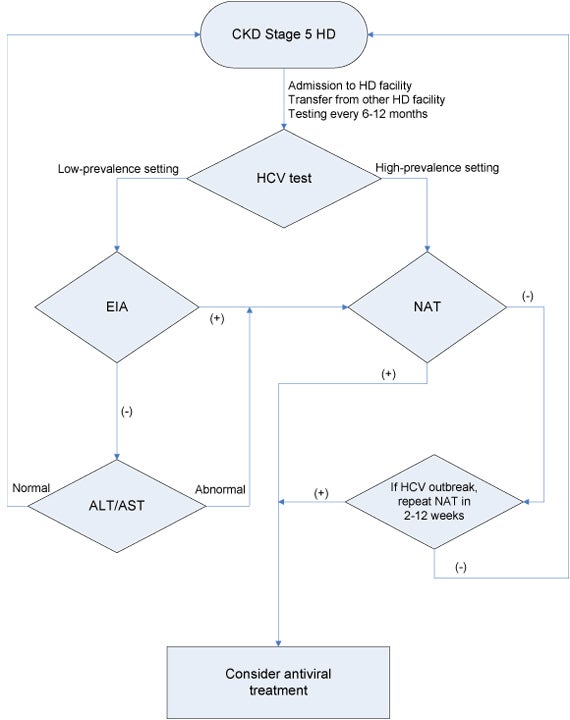
Algorithm 1: CKD stage 5 hemodialysis diagnostic algorithm (Guideline 1.2) Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CKD, chronic kidney disease; EIA, enzyme immunoassay; HCV, hepatitis C virus; NAT, nucleic acid test.
In the setting of suspected nosocomial HCV infection in an HD facility, testing with NAT should be performed in all patients who may have been exposed (strong evidence* G 1.2.4). Repeat testing with NAT is suggested for initially NAT-negative patients within 2-12 weeks because of the risk of false negative NAT testing early after infection (weak evidence* G 1.2.4).
Treatment of HCV infection in Hemodialysis
- Evaluate HCV-infected patients for antiviral treatment (weak evidence G.2.1.1)
- Life expectancy
- Candidacy for kidney transplantation
- Comorbidities such as cardiovascular disease (weak evidence G.2.1.2).
- Use Interferon (IFN) Monotherapy in HD Patients (weak evidence G. 2.2.3)
- Monitor the response to antiviral therapy (G.2.3)
Treat HCV based on the potential benefits and risks of therapy, including:
Start antiviral treatment if the HCV infection is acute. A waiting period beyond 12 weeks to observe spontaneous clearance (by NAT) is not justified (weak evidence G.2.1.3).
Consider antiviral therapy for patients with HCV-related glomerulonephritis (GN) (weak evidence G.2.1.6).
IFN
Alfa-2a IFN: 3mU SQ 3 times per week
Alfa-2b IFN: 3mU SQ 3 times per week
Patients with HCV genotypes 1 and 4 should receive 48 weeks of IFN therapy if an early viral response is obtained at 12 weeks (>2 log fall in viral titer).
Patients with genotypes 2 and 3 should be treated for 24 weeks.
Tolerance to IFN therapy is suggested to be lower in maintenance HD patients than in non-CKD patients infected with HCV
Adverse Effects of IFN
Headache
Flu-like illness
Depression
Neurologic and cardiovascular disorders
Note: Ribavirin is NOT recommended for use in patients receiving HD.
Absolute Contraindications to IFN Therapybr.
Pregnancy
Breastfeeding
Some Relative Contraindications to IFN Therapy4
Decompensated liver disease
Major neuropsychiatric disease
Coronary or cerebrovascular disease
Poorly controlled diabetes
Obstructive pulmonary disease
Active substance or alcohol abuse
History of kidney or heart transplantation
All patients >60 years of age are in a higher risk group for the development of serious adverse reactions to IFN and require individual decision-making.
How should response be assessed?
Use sustained virologic response (SVR), defined as HCV RNA clearance 6 months after completion of antiviral treatment (weak evidence G.2.3.1).
When should further monitoring occur?
If SVR is achieved, repeat test with NAT every 6 months to ensure patient remains nonviremic (weak evidence G.2.3.2).
Which patients with HCV infection should be followed for HCV-associated comorbidities?
- All patients, regardless of treatment or response (strong evidence G.2.3.3).
- For patients with evidence of clinical or histologic cirrhosis, evaluate very 6 months (strong evidence G.2.3.3).
Hemodialysis Units: Preventing HCV Transmission (Guideline 3)
Hemodialysis units have responsibility to ensure implementation of, and adherence to, strict infection-control procedures designed to prevent nosocomial transmission of blood-borne pathogens, including HCV (strong evidence G.3.1) between patients in their care, either directly or via contaminated equipment or surfaces (see Tables 1 and 2).
Regular observational audits of infection-control procedures are suggested for inclusion in performance reviews of hemodialysis units (weak evidence G.3.2).
- Center-wide issues to consider:
- Dialysis unit design should facilitate implementation of infection control strategies
- Time between shifts should be sufficient to enable effective machine and surface decontamination
- Strategically position gloves around the unit to facilitate quick access
- Ease of disinfection should be a consideration when selecting new equipment
- Ensure that infection-control staff training and vigilance is maintained during changes to staff-to-patient ratios or employment of new staff
- Carry out regular risk assessments and develop procedures to reduce or remove hazards
- Isolating HCV-infected patients as an alternative to strict infection-control procedures for preventing transmission of blood-borne pathogens (weak evidence G.3.1).
- Using dedicated dialysis machines for HCV-infected patients (moderate evidence G.3.1).
- Dialyzers of HCV-infected patients can be reused provided there is implementation of, and adherence to, strict infection-control procedures (weak evidence G.3.1).
Not Recommended:
When dialyzer reuse is unavoidable:

Table 1. Hygienic Precautions for Hemodialysis (General)
Definitions
A "dialysis station" is the space and equipment within a dialysis unit that is dedicated to an individual patient. This may take the form of a well-defined cubicle or room, but there is usually no material boundary separating dialysis stations from each other or from the shared areas of the dialysis unit.
A "potentially contaminated" surface is any item of equipment at the dialysis station that could have been contaminated with blood, or fluid containing blood since it was last disinfected, even if there is no evidence of contamination.
Education
A program of continuing education covering the mechanisms and prevention of cross infection should be established for staff caring for hemodialysis patients. Appropriate information on infection control should also be given to nonclinical staff, patients, caregivers and visitors.
Hand Hygiene
Staff should wash their hands with soap or an antiseptic hand-wash and water, before and after contact with a patient or any equipment at the dialysis station. An antiseptic alcohol gel rub may be used instead when their hands are not visibly contaminated.
In addition to hand washing, staff should wear disposable gloves when caring for a patient or touching any potentially contaminated surfaces at the dialysis station. Gloves should always be removed when leaving the dialysis station.
Where practical, patients should also clean their hands, or use an alcohol gel rub, when arriving at and leaving the dialysis station.
Equipment Management (for management of the dialysis machine, see Table 2)
Single-use items required in the dialysis process should be disposed of after use on one patient.
Nondisposable items should be disinfected after use on one patient. Items that cannot be disinfected easily (for example, adhesive tape, tourniquets) should be dedicated to a single patient.
The risks associated with the use of physiologic monitoring equipment (e.g., blood pressure monitors, weight scales, access flow monitors) for groups of patients should be assessed and minimized. Blood pressure cuffs should be dedicated to a single patient or made from a light-colored, wipe-clean fabric.
Medications and other supplies should not be moved between patients. Medications provided in multiple-use vials, and those requiring dilution using a multiple-use diluent vial, should be prepared in a dedicated central area and taken separately to each patient. Items that have been taken to the dialysis station should not be returned to the preparation area.
After each session, all potentially contaminated surfaces at the dialysis station should be wiped clean with a low-level disinfectant if not visibly contaminated. Surfaces that are visibly contaminated with blood or fluid should be disinfected with a commercially available tuberculocidal germicide or a solution containing at least 500 p.p.m. hypochlorite (a 1:100 dilution of 5% household bleach).
Waste Management
Needles should be disposed of in closed, unbreakable containers that should not be overfilled. A "no-touch" technique should be used to drop the needle into the container, as it is likely to have a contaminated surface. If this is difficult due to the design of the container, staff should complete patient care before disposing of needles.
The used extracorporeal circuit should be sealed as effectively as possible before transporting it from the dialysis station in a fluid-tight waste bag or leak-proof container. If it is necessary to drain the circuit, or to remove any components for reprocessing, this should be done in a dedicated area away from the treatment and preparation areas.
Table 2. Hygienic Precautions for Hemodialysis (Dialysis Machines)
Definitions
The "transducer protector" is a filter (normally a hydrophobic 0.2 mm filter) that is fitted between the pressure monitoring line of the extracorporeal circuit and the pressure-monitoring port of the dialysis machine. The filter allows air to pass freely to the pressure transducer that gives the reading displayed by the machine, but it resists the passage of fluid. This protects the patient from microbiologic contamination (as the pressure monitoring system is not disinfected) and the machine from ingress of blood or dialysate. An external transducer protector is normally fitted to each pressure monitoring line in the blood circuit. A back-up filter is located inside the machine. Changing the internal filter is a technical job.
A "single-pass machine" is a machine that pumps the dialysate through the dialyzer and then to waste. In general, such machines do not allow fluid to flow between the drain pathway and the fresh pathway except during disinfection. "Recirculating" machines produce batches of fluid that can be passed through the dialyzer several times.
Transducer Protectors
External transducer protectors should be fitted to the pressure lines of the extracorporeal circuit. Before commencing dialysis, staff should ensure that the connection between the transducer protectors and the pressure-monitoring ports is tight, as leaks can lead to wetting of the filter.
Transducer protectors should be replaced if the filter becomes wet, as the pressure reading may be affected. Using a syringe to clear the flooded line may damage the filter and increase the possibility of blood passing into the dialysis machine.
If wetting of the filter occurs after the patient has been connected, the line should be inspected carefully to see if any blood has passed through the filter. If any fluid is visible on the machine side, the machine should be taken out of service at the end of the session so that the internal filter can be changed and the housing disinfected.
External Cleaning
After each session, the exterior of the dialysis machine should be cleaned with a low-level disinfectant if not visibly contaminated.
If a blood spillage has occurred, the exterior should be disinfected with a commercially available tuberculocidal germicide or a solution containing at least 500 p.p.m. hypochlorite (a 1:100 dilution of 5% household bleach) if this is not detrimental to the surface of dialysis machines. Advice on suitable disinfectants, and the concentration and contact time required, should be provided by the manufacturer.
If blood or fluid is thought to have seeped into inaccessible parts of the dialysis machine (for example, between modules, behind blood pump), the machine should be taken out of service until it can be dismantled and disinfected.
Disinfection of the Internal Fluid Pathways
It is not necessary for the internal pathways of a single-pass dialysis machine to be disinfected between patients, unless a blood leak has occurred, in which case both the internal fluid pathways and the dialysate-to-dialyzer (Hansen) connectors should be disinfected before the next patient. If machines are not subjected to an internal disinfection procedure, staff should ensure that sufficient time is available between patients for the external surfaces to be disinfected. Machines with recirculating dialysate should always be put through an appropriate disinfection procedure between patients.
Disclaimer
SECTION I: USE OF THE CLINICAL PRACTICE GUIDELINES
These Clinical Practice Guidelines are based on the best information available at the time of publication. They are designed to provide information and assist decision-making. They are not intended to define a standard of care and should not be construed as one, nor should they be interpreted as prescribing an exclusive course of management. Variations in practice will inevitably and appropriately occur when clinicians take into account the needs of individual patients, available resources and limitations unique to an institution or a type of practice. Every health care professional making use of these guidelines is responsible for evaluating the appropriateness of applying them in the setting of any particular clinical situation. The recommendations for research contained within this document are general and do not imply a specific protocol.
SECTION II: DISCLOSURE
Kidney Disease Outcomes Quality Initiative (KDOQI) makes every effort to avoid any actual or reasonably perceived conflicts of interest that may arise as a result of an outside relationship or a personal, professional or business interest of a member of the Work Group. Specifically, all members of the Work Group are required to complete, sign, and submit a disclosure and attestation form showing all such relationships that might be perceived as actual or perceived conflicts of interest. This document is updated annually and information is adjusted accordingly. All reported information is on file at the National Kidney Foundation.
References
- National Kidney Foundation. KDIGO clinical practice guidelines for the prevention, diagnosis, evaluation, and treatment of hepatitis C in chronic kidney disease. Kidney International. 2008 (suppl 1); 73:S1-S99.
- Alter MJ. Epidemiology of hepatitis ? in the West. Semin Liver Dis. 1995 15-5-14.
- Management of hepatitis C. NIH Consensus Statement. 1997 March 24-26,15(3)
- World Health Organization. Hepatitis C prevention and treatment. Available at http://www.who.int/csr/disease/hepatitis. Accessed on October 3, 2008.
- KDOQI U.S. commentary on the KDIGO clinical practice guideline for the prevention, diagnosis, evaluation, and treatment of hepatitis C in CKD.