Hepatitis C virus infection can cause liver issues and accelerate CKD progression. Learn about HCV's impact on CKD, screening methods, and the latest treatment options for better management.
Hepatitis C
Hepatitis C virus infection (HCV) is associated with increased risk of liver fibrosis or cirrhosis and development of hepatocellular carcinoma.1,2 HCV infection is also a common indication for liver transplant in the United States.
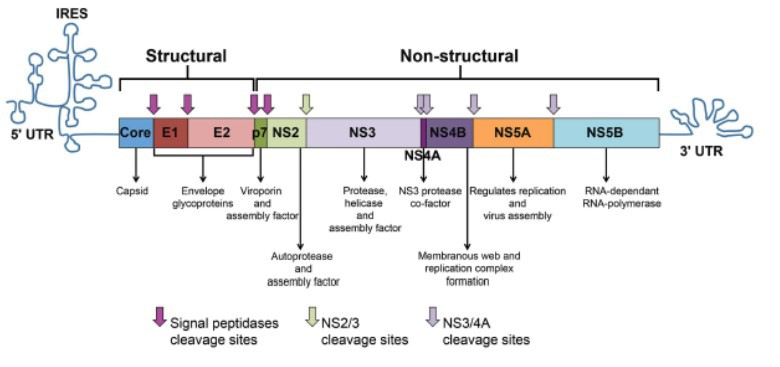
The HCV genome is a single-stranded, positive sense RNA strand with a nucleotide length of 9.6 kb, and encodes for a single polypeptide (Figure 1).3 The virus is transmitted through blood and infects hepatocytes by interacting with co-receptors that results in its endocytosis, followed by fusion of the viral genome with the endosome and uncoating of its RNA, which is then cleaved into 10 viral proteins (3 structural and 7 nonstructural). The nonstructural HCV proteins (P7, NS2, NS3, NS4A, NS4B, NS5A, and NS5B) are processed by both viral and host proteases, and are essential in the HCV life cycle.4,5,6 There are at least six HCV genotypes (GT 1 - 6).
An estimated 2.7 - 3.9 million people in the United States have chronic infection of the hepatitis C virus. The majority (75% - 85%) of newly infected individuals will develop chronic infection.1,2
Hepatitis C and CKD
HCV infection is a recognized cause of progression to kidney failure, and is associated with reduced survival in the CKD population.7,8 A study by Molnar et al found that HCV infection is associated with a higher mortality risk, incidence of decreased kidney function, and progressive loss of kidney function.9 Multivariate adjusted models showed HCV infection independently was associated with a 2.2 - fold higher mortality (adjusted hazard ratio (aHR), 95% confidence interval (CI): 2.13; 2.21), and a 15% higher incidence of decreased kidney function (aHR, 95% CI: 1.12; 1.17).
The prevalence of HCV infection is higher among CKD patients of all stages compared with rates in the general population, but is particularly high among patients receiving hemodialysis.10,11,12 HCV infection is a concern in kidney transplantation, as it has been implicated in diminished patient and graft survival.13 There is also an association between chronic HCV infection and glomerular diseases, including mixed cryoglobulinemia, membranoproliferative glomerulonephritis (MPGN), membranous nephropathy, and polyarteritis nodosa (PAN).14,15,16
Screening and Testing
Diagnostic tests for HCV consist of two broad categories: (1) serologic assays that detect antibodies to HCV and (2) molecular assays that detect or quantify HCV RNA. Other diagnostic modalities (e.g., genotype testing, serum fibrosis panels, and liver biopsy) can help predict prognosis and help select the optimal treatment regimen.17 A testing sequence for identifying current HCV infection, consisting of initial HCV antibody testing (either rapid or laboratory- conducted assay) followed by an HCV RNA assay for all positive antibody tests, has been recommended.1,8,18
HCV testing of patients with CKD should be performed in patients with unexplained proteinuria, microscopic hematuria, increased aminotransferase (ALT) levels, or other known risk factors for HCV acquisition.8,19 All transplant candidates are also tested. Serologic testing to detect HCV antibodies is suggested for non-dialysis CKD and hemodialysis in units with low prevalence of HCV. A positive result should be followed by molecular testing to detect HCV RNA in the blood - qualitative testing to detect the presence of HCV RNA, and quantitative testing to detect the viral load.
Society guidelines recommend screening patients at increased risk for HCV with known risk factors for HCV acquisition.1,18,20 Factors that should prompt at least one-time testing for HCV in the general population include the following:1,20
- Born from 1945 through 1965
- Injected illicit drugs
- Clotting factor concentrates made prior to 1987
- Blood transfusions or solid organ transplants prior to July 1992
- Long-term hemodialysis treatment
- Healthcare-related exposure to HCV-infected blood
- Recipients of blood or organs from a donor later testing HCV positive
- HIV infection
- Signs or symptoms of liver disease (e.g., abnormal liver enzyme tests)
- Children born to HCV-positive mothers (test after age 18 months of birth)
- Receiving a tattoo in an unregulated setting
- Incarceration
Management of HCV and CKD
The goal of treatment is to achieve sustained virologic response (SVR) (defined as undetectable HCV RNA 12 weeks post-treatment). Optimal treatment for HCV-infected CKD patients should consider the HCV genotype, extent of liver damage, CKD stage, transplant candidacy, prior HCV treatment, patient comorbidities, and the benefits and risks of antiviral treatment itself.8
Interferon-based therapy was used sparingly in the past as it was limited by low efficacy as well as high toxicity. A meta-analysis of 10 clinical studies by Fabrizi et al studying interferon/ribavirin (IFN/RBV) in HCV-infected patients receiving hemodialysis showed a summary SVR rate of 56% and discontinuation rate of 25%.21
The advent of direct-acting antivirals (DAAs) has dramatically changed the approach to HCV genotype and viral load. For example, SVR rates generally exceed 90% in most subpopulations with DAAs.22,23 These therapies target non-structural parts of the HCV genome and include NS3/4A protease inhibitors, nucleotide analog NS5B polymerase inhibitors, NS5A inhibitors, and multi-class combination antiviral medications. These newer therapies have been the focus of clinical research in recent years for their improved efficacy, compared to interferon-based therapies. Typically, multiple DAAs are used in combination, NS5A or NS5B inhibitor with a protease inhibitor, to target the different aspects of the HCV genome. Multi-class combination antiviral medications for HCV infection are summarized in Figure 2.
-Grazoprevir: NS3/4A protease inhibitor
4
-Grazoprevir: Hepatic
-Pibrentasvir: NS5A inhibitor
-Pibrentasvir: Hepatic
-Paritaprevir: NS3/4A protease inhibitor
-Ritonavir: CYP3A inhibitor
-Dasabuvir: NS5B polymerase inhibitor
-Paritaprevir: Hepatic
-Ritonavir: Hepatic
-Dasabuvir: Hepatic
-Paritaprevir: NS3/4A protease inhibitor
-Ritonavir: CYP3A inhibitor
-Paritaprevir: Hepatic
-Ritonavir: Hepatic
-Sofosbuvir: NS5B polymerase inhibitor
1,4,5, or 6
-Sofosbuvir: Renal
-Velpatasvir: NS5A inhibitor
-Velpatasvir: Hepatic
-Velpatasvir: NS5A inhibitor
-Voxilaprevir: HCV NS3/4A protease inhibitor
-Velpatasvir: Hepatic
-Voxilaprevir: Hepatic
*Consult Package Insert for specific indications24
†Not licensed for use in patients with a GFR <30 mL/min/1.73m2
Determining HCV genotype is still a part of defining the hepatitis C treatment regimen for a patient, as the efficacy of several DAAs can vary by genotype. With the recent arrival of several pan-genotypic DAAs, it is possible that determining genotype may have a lesser role in planning HCV treatment in the future.
Among the currently approved DAAs, sofosbuvir is the only one that has significant renal elimination. Other currently approved DAAs (Figure 2) are not eliminated by the kidneys. Sofosbuvir-containing regimens are not licensed for use in patients with a GFR <30 mL/min/1.73m2 so its use in advanced CKD is off label and more information about its toxicity in this setting is needed before its use can be expanded in this population.25,26,27
Studies have demonstrated effective use of DAAs in CKD. For example, the C-SURFER trial evaluated elbasvir-grazoprevir in patients with HCV GT 1 infection and advanced CKD (stages 4 and 5, including patients on hemodialysis) with or without liver cirrhosis. The study showed that elbasvir-grazoprevir achieved an SVR12 in 94% of patients in the primary intent-to-treat (ITT) analysis.20,28,29 The EXPEDITION-4 study showed that glecaprevir-pibrentasvir achieved an SVR12 of 98% (primary ITT) in HCV-infected patients with advanced CKD.30 The study included treatment-naive and -experienced patients (previous IFN or pegIFN ± RBV, or sofosbuvir and ribavirin ± pegIFN) with CKD stage 4 or 5 (including hemodialysis).
The field of HCV treatment in CKD stages 4–5 has changed drastically. Research has focused on newer treatment options, including pan-genotypic, multi-class combination DAAs. HCV cure is now possible in most cases. This offers an important opportunity to further reduce the prevalence of HCV in hemodialysis, and thus reduce further the risk of nosocomial transmission of HCV in hemodialysis units.25 There are other areas of research aimed at improving outcomes and addressing unmet medical needs, such as the utility and safety of HCV positive donor organs, which have the potential to help broaden supply, and thus, shorten wait times.31 This strategy can be made more possible due to the 90-100% SVR rates with newer DAA regimens, and potentially shorter time to transplantation in those given a HCV positive graft.25
Hepatitis C Treatment in CKD: The KDIGO Perspective25
- In patients with an eGFR >30 mL/min/1.73m2, all licensed DAAs regimens can be used.
- Cure of HCV appears at hand in CKD stages 4–5, including dialysis patients, and in kidney transplant recipients.
- The choice of DAA regimen in CKD should be based on HCV genotype, viral load, eGFR, concomitant medications, transplant candidacy and comorbidities.
- The timing of treatment in potential kidney transplantation candidates (before versus after transplantation) should be decided in collaboration with the nephrologist and transplant center.
References
- Centers for Disease Control and Prevention. Hepatitis C information. www.cdc.gov/hepatitis/hcv/index.htm. Accessed January 15, 2018.
- Denniston M, Jiles R, Drobeniuc J, et al. Chronic hepatitis C virus infection in the United States,
National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160:293-300. - Abdel-Hakeem M, Shoukry N. Protective immunity against hepatitis C: many shades of gray.
Front Immunol. 2014;5:274. - Chevaliez S, Pawlotsky J-M. HCV genome and life cycle. In: Tan S-L, ed. Hepatitis C viruses: genomes and molecular biology. Norfolk, UK: Horizon Bioscience; 2006.
- Tencate V, Sainz B Jr., Cotler S, Uprichard S. Potential treatment options and future research to increase hepatitis C virus treatment response rate. Hepat Med. 2010;2:125-145.
- Thompson R. Emerging therapeutic options for the management of Hepatitis C infection. World J Gastroenterol. 2014;20:7079-7088.
- Perico N, Cattaneo D, Bikbov B, Remuzzi G. Hepatitis C infection and chronic renal diseases. Clin J Am Soc Nephrol. 2009;4:207-220.
- Kidney Disease: Improving Global Outcomes (KDIGO). KDIGO clinical practice guidelines for the prevention, diagnosis, evaluation, and treatment of hepatitis C in chronic kidney disease. Kidney Int. 2008;73(Suppl 109):S1-S99.
- Molnar M, Alhourani H, Wall B, et al. Association of hepatitis C viral infection with incidence and progression of chronic kidney disease in a large cohort of US veterans. Hepatology. 2015;61:1495-1502.
- Kalantar-Zadeh K, Miller L, Daar E. Diagnostic discordance for hepatitis C virus infection in hemodialysis patients. Am J Kidney Dis. 2005;46:290-300.
- Kalantar-Zadeh K, Kilpatrick R, McAllister C, et al. Hepatitis C virus and death risk in hemodialysis patients. J Am Soc Nephrol. 2007;18:1584-1593.
- Goodkin D, Bieber B, Jadoul M, et al. Mortality, hospitalization, and quality of life among patients with hepatitis C infection on hemodialysis. Clin J Am Soc Nephrol. 2017;12:287-297.
- Fabrizi F, Martin P, Dixit V, et al. Meta-analysis of observational studies: Hepatitis C and survival after renal transplant. J Viral Hepat. 2014;21:314-324.
- Kamar N, Rostaing L. Glassock R, Hirsch M, Lam A (Eds). Overview of renal disease associated with hepatitis C virus infection. UptoDate, Waltham MA. Accessed January 15, 2018.
- McGuire B, Julian B, Bynon J, et al. Glomerulonephritis in patients with hepatitis C virus cirrhosis undergoing liver transplantation. Ann Intern Med. 2006;144:735.
- Tarantino A, Campise M, Banfi G, et al. Long-term predictors of survival in essential mixed cryoglobulinemic glomerulonephritis. Kidney Int. 1995;47:618.
- Terrault N, Chopra S. Di Bisceglie A, Bloom A (eds.). Diagnosis and evaluation of chronic hepatitis C virus infection. UptoDate, Waltham MA. Accessed January 15, 2018.
- Centers for Disease Control and Prevention. Testing for HCV infection: an update of guidance for clinicians and laboratorians. MMRW. 2013;62:362-365.
- Gordon C, Balk E, Becker B, et al. KDOQI US commentary on the KDIGO clinical practice guideline for the prevention, diagnosis, evaluation, and treatment of hepatitis C in CKD. Am J Kidney Dis. 2008;52:811-825.
- American Association for the Study of Liver Diseases, and the Infectious Diseases Society of America (AASLD-IDSA). Recommendations for testing, managing, and treating hepatitis C. http://www.hcvguidelines.org. Accessed January 15, 2018.
- Fabrizi F, Dixit V, Martin P, et al. Combined antiviral therapy of hepatitis C virus in dialysis patients: meta-analysis of clinical trials. J Viral Hepat. 2011;18:e263-e269.
- Pockros P. Di Bisceglie A, Bloom A (Eds). Direct-acting antivirals for the treatment of hepatitis C virus infection. UptoDate, Waltham MA. Accessed January 15, 2018.
- Seifert L, Perumpail R, Ahmed A. Update on hepatitis C: Direct-acting antivirals. World J Hepatol. 2015;7:2829-2833.
- Package Inserts: Elbasvir-Grazoprevir (Zepatier), Glecaprevir-Pibrentasvir (Mavyret), Ledipasvir-Sofosbuvir (Harvoni), Ombitasvir-Paritaprevir-Ritonavir and Dasabuvir (Viekira Pak), Ombitasvir-Paritaprevir-Ritonavir (Technivie), Sofosbuvir-Velpatasvir (Epclusa), Sofosbuvir- Velpatasvir-Voxilaprevir (Vosevi). Accessed January 15, 2018
- Jadoul M, Martin P. Hepatitis C treatment in chronic kidney disease patients: The Kidney Disease Improving Global Outcomes perspective. Blood Purif. 2017;43:206-209.
- Kwo P, Badshah M. New Hepatitis C virus therapies: drug classes and metabolism, drug interactions relevant in the transplant settings, drug options in decompensated cirrhosis, and drug options in end-stage renal disease. Curr Opin Organ Transplant. 2015;20:235-241.
- Bhamidimarri K, Martin P. Finally, safe and effective treatment options for Hepatitis C in hemodialysis patients. J Hepatol. 2016;65:7-10.
- Roth D, Nelson D, Bruchfeld A, et al. Grazoprevir plus elbasvir in treatment-naive and treatment-experienced patients with Hepatitis C virus genotype 1 infection and stage 4-5 chronic kidney disease (the C-SURFER study): a combination phase 3 study. Lancet. 2015;386:1537-1545.
- Bhamidimarri. K Grazoprevir plus elbasvir and other treatment options in Hepatitis C infected patients with stage 4–5 chronic kidney disease. Ann Transl Med. 2016;4(Suppl 1):S13.
- Gane E, Lawitz E, Pugatch D, et al. EXPEDITION-4: Efficacy and safety of glecaprevir/pibrentasvir (ABT-493/ABT-530) in patients with renal impairment and chronic Hepatitis C virus genotype 1-6 infection. American Association for the Study of Liver Diseases (AASLD). The Liver Meeting. Boston, MA. November 2016.
- Reese P, Abt P, Blumberg E, et al. Transplanting Hepatitis C-positive kidneys. N Engl J Med. 2015;373:303-305.

















